Biotech NewswireAugust 20, 2025
Tag: Neurizon , biotech company , NUZ-001 , ALS
The OLE study met its primary endpoint, confirming long-term treatment with NUZ-001 at the recommended Phase 2 dose was safe and well-tolerated
Treatment during the OLE was associated with a durable functional benefit (ALSFRS-R decline –0.88 points/month), minimal respiratory decline (stable SVC), and supportive biomarker trends (stable plasma NfL and decreased (–17%) urinary p75ECD)
Preliminary efficacy was assessed by comparing NUZ-001-treated patients to matched, untreated historical controls from the PRO-ACT database from the start of the Phase 1 MEND Study:
NUZ-001 significantly increased survival (χ2=13.75, p=0.00021)
NUZ-001 significantly reduced the risk of death by 76.7% (HR=0.233, p=0.0013)
Treatment with NUZ-001 showed an estimated median survival extension of ~16 months
NUZ-001 slowed the rate of functional decline by 31% (–0.83 vs –1.21 ALSFRS-R points/month; p = 0.145)
NUZ-001 reduced the rate of respiratory decline by 43% (–1.65 vs -2.93 VC PP points/month; p=0.078)
NUZ-001 has been safely used for more than 2.5 years, with five patients still receiving treatment under the TGA’s Special Access Scheme
These encouraging results reinforce the rationale for the planned pivotal HEALEY ALS Platform Trial, expected to commence in Q4 CY2025
Neurizon? Therapeutics Limited (ASX: NUZ & NUZOA) (“Neurizon” or “the Company”), a clinical-stage biotech company dedicated to advancing innovative treatments for neurodegenerative diseases, is pleased to report positive topline results from the Open-Label Extension (OLE) study of its lead candidate NUZ-001, for the treatment of amyotrophic lateral sclerosis (ALS), the major form of motor neurone disease (MND).
Long-term treatment with NUZ-001 at the recommended Phase 2 dose was safe and well-tolerated. Importantly, preliminary efficacy findings demonstrated that treatment with NUZ-001, compared to matched historical controls from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database, was associated with a significant survival advantage, sustained slowing of global functional decline, a reduced rate of respiratory decline, and a stable or downward trend in key disease biomarkers.
These encouraging results support the potential of NUZ-001 as a disease-modifying therapy for ALS and provide strong justification for advancing the program into further clinical development.
Managing Director and Chief Executive Officer, Dr. Michael Thurn, commented: “We are very encouraged by these long-term treatment results, which reinforce the potential of NUZ-001 to deliver meaningful clinical benefits for people living with ALS. Importantly, the therapy has been shown to be safe and well-tolerated, even with extended use. For too long, patients and families have faced this devastating disease with very few treatment options, and the field has struggled to find truly viable new options. To see sustained functional and respiratory benefits, a clear survival advantage, and supportive biomarker trends after nearly three years of treatment gives us additional confidence as we finalise preparations for entry into the HEALEY ALS Platform Trial. I would like to thank Associate Professor Susan Mathers at Calvary Health Care Bethlehem and Professor Dominic Rowe at Macquarie University, as well as their clinical teams and the incredible patients, families, and caregivers who made this study possible.”
The OLE study was an open-label, safety, tolerability, and preliminary efficacy study of NUZ-001 in 10 patients with ALS who completed the Phase 1 MEND Study, at the recommended Phase 2 dose of 10 mg/kg administered orally daily for 12-months.
Daily treatment with NUZ-001 was safe and well-tolerated, with no treatment-related deaths observed. Eight patients completed the study, while two patients died during the OLE due to respiratory failure and pneumonia. Throughout the study, a total of 25 treatment-emergent adverse events (AEs) were reported, of which only 3 were considered possibly treatment-related (mild to moderate dry mouth at night, increased hair growth, and elevated liver enzymes). Notably, there were no treatment-related severe AEs and no discontinuations due to AEs.
Five patients continue to access NUZ-001 via the Therapeutic Goods Administration (TGA) Special Access Scheme (SAS). Since the Phase 1 MEND Study started in October 2022, the initial patients enrolled have now been on daily treatment with NUZ-001 for over 2.5 years, providing strong evidence of the excellent long-term safety profile of NUZ-001.
During the OLE study, disease progression was assessed using the FDA-accepted primary endpoint for ALS trials, the ALS Functional Rating Scale-Revised (ALSFRS-R). Among the 10 patients, the estimated mean rate of decline was -0.88 points/month (CI: -1.58, 0.10), compared with -0.74 points/month (CI: -1.26, -0.23) observed in the Phase 1 MEND Study. These comparable results indicate a stable and durable treatment effect with long-term NUZ-001 therapy.
Slow Vital Capacity (SVC) decline is a validated marker of disease progression and survival in ALS. In the OLE study, the mean Vital Capacity Percent Predicted (VC PP) at baseline was 72.4% (CI: 54.41, 90.45), decreasing gradually to 62.33% (CI: 36.69, 87.96) at month 12. This 12-month value was not statistically different from the baseline value in the Phase 1 MEND Study of 84.40% (CI: 67.94, 100.86; p = 0.3616) despite long-term treatment. These findings are consistent with a durable treatment effect and support the potential disease-modifying activity of NUZ-001 in ALS.
The results of the additional exploratory efficacy measures of ALSSQOL-R quality of life scores and ECAS further supported a durable treatment effect despite long-term treatment with NUZ-001. In addition, plasma NfL levels remained broadly stable over the 12-month OLE, with a transient reduction observed at Month 6 before returning to baseline levels by Month 12 (n=7), while urinary p75ECD decreased by ~17% (n=3). These stable and downward biomarker trends over the OLE period provide additional evidence supporting the potential disease-modifying effect of NUZ-001.
The PRO-ACT database is the largest publicly available dataset for ALS clinical trial data worldwide. The richness of the PRO-ACT lies in its longitudinal clinical, laboratory, and outcome data. Because of its size and breadth, PRO-ACT has become a critical external control resource for ALS drug development. In early-phase studies patient trajectories from PRO-ACT can serve as a virtual placebo or reference group, against which the observed treatment (ALSFRS-R slope, SVC decline, and survival) effect can be benchmarked.
A comparative analysis against the PRO-ACT database was conducted for the entire treatment period encompassing both the Phase 1 MEND and OLE studies, reflecting continuous NUZ-001 administration. Figures 1 and 2 present the ALSFRS-R and SVC decline, respectively, for all 12 patients. The matching protocol model used months since onset, pre-baseline slope, onset site, and baseline ALSFRS-R as fixed effects to align historical control patients with those treated with NUZ-001.
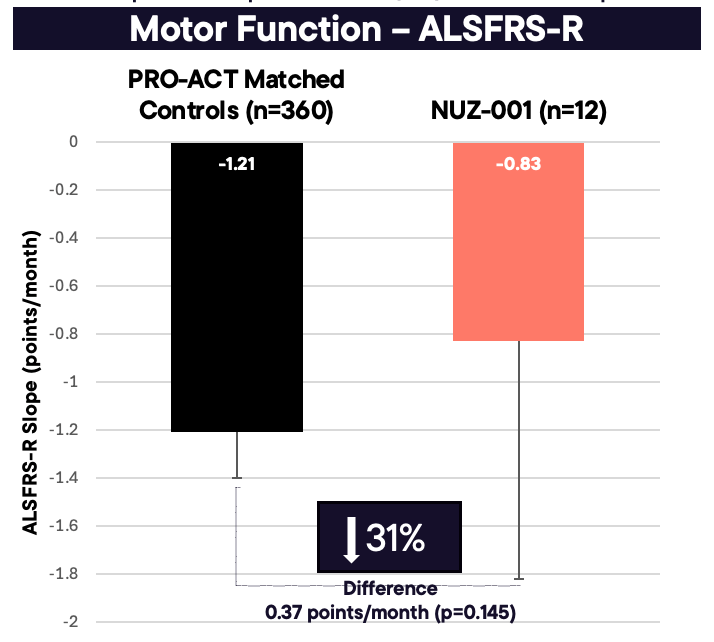
Figure 1: Slowing in ALSFRS-R Decline
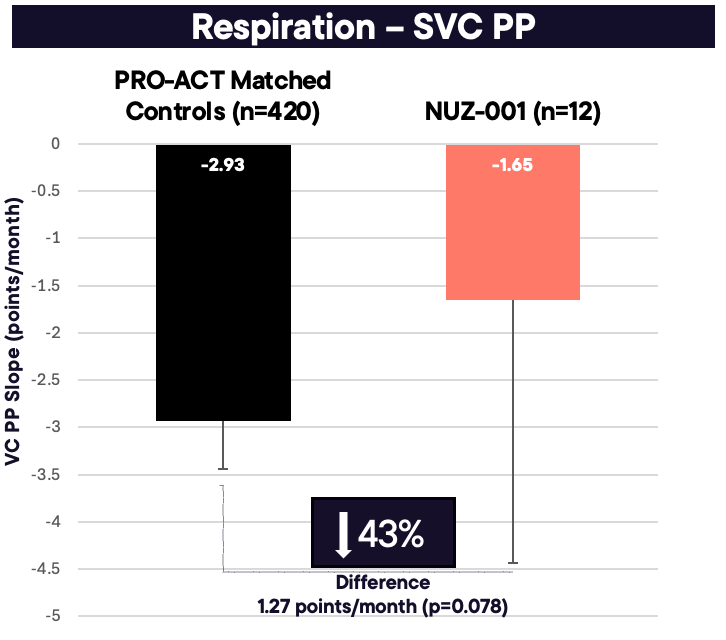
Figure 2:Slowing in VC PP Decline
As shown in Figure 1, compared with untreated matched historical controls from the PRO-ACT database, patients treated with NUZ-001 exhibited a 0.37 points/month slower decline in ALSFRS-R, corresponding to a 31% reduction in disease progression (–0.83 vs –1.21 points/month; p = 0.145). The estimated rate of decline in VC PP for all 12 NUZ-001–treated patients was –1.65 points/month, compared with –2.93 points/month for untreated matched controls (difference: 1.27; p = 0.078; see Figure 2). This represents a 43% slowing in VC PP decline, which aligns closely with the 31% slowing observed in ALSFRS-R, further supporting the potential disease-modifying effect of NUZ-001.
As of 15 August 2025, 6 of the 12 patients who participated in the initial Phase 1 MEND study remain alive. Treatment durations range from 11.3 to 34.4 months (median 28.9 months). In the most conservative dataset, the log-rank test shows a significant survival benefit (χ2 = 13.75, p = 0.00021). The Cox proportional hazards analysis estimated a hazard ratio of 0.233 (95% CI: 0.096, 0.566, p = 0.0013), corresponding to a 76% reduction in the risk of death versus untreated matched controls and an estimated median survival extension of ~16 months.
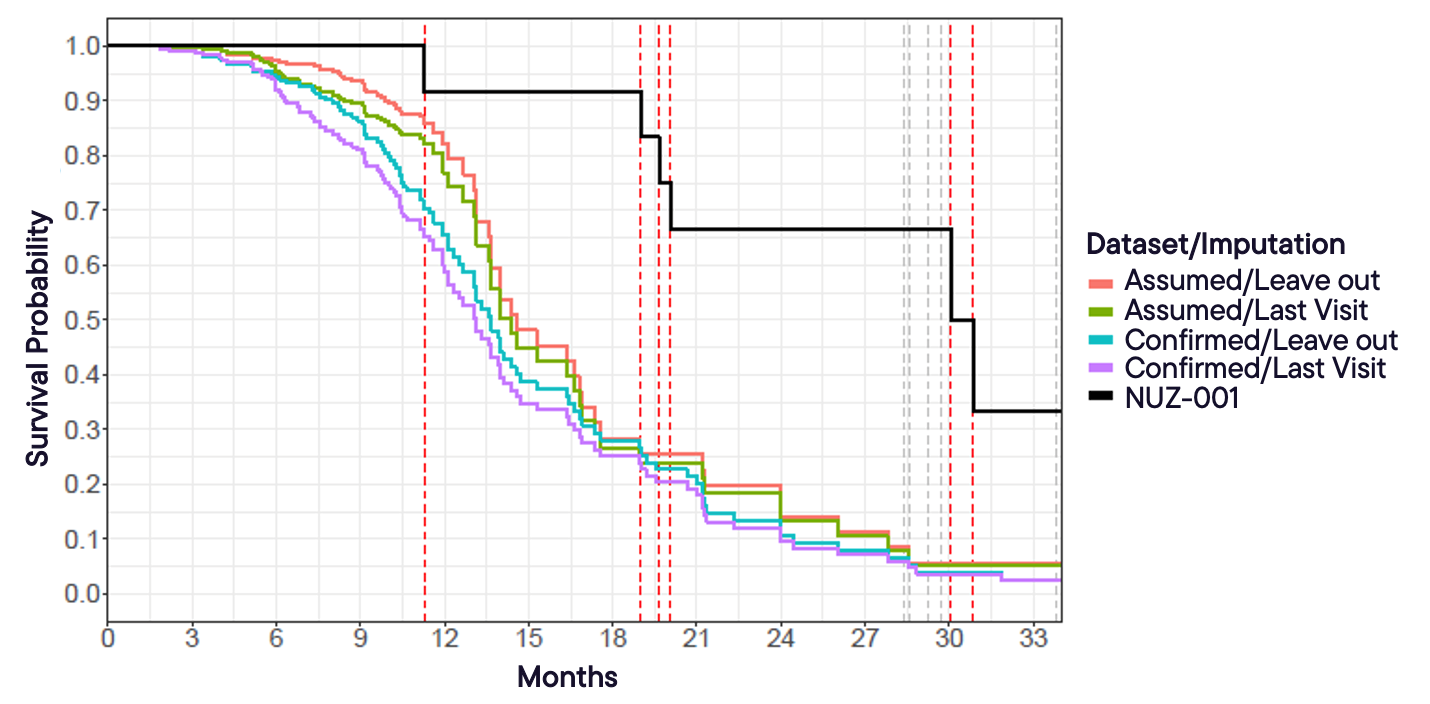
Figure 3: Survival Probability Analysis
In Figure 3, estimated Kaplan-Meier curves are shown for the NUZ-001 treatment group and each of the matched PRO-ACT control datasets described in Table 2. In these analyses, the “Assumed” Dataset treats missing death status as indicating survival, whereas the “Confirmed” dataset includes only patients’ non-missing death data. The imputation method “Leave out” excludes missing times of death for confirmed deceased patients, while “Last Visit” assigns the last visit date as the time of death. Grey vertical dashed lines indicate exposure time, and the red vertical dashed lines represent death events.
This figure highlights how estimated survival probabilities vary depending on the dataset and imputation method, with the “Assumed” dataset using “Leave out” producing higher survival estimates.
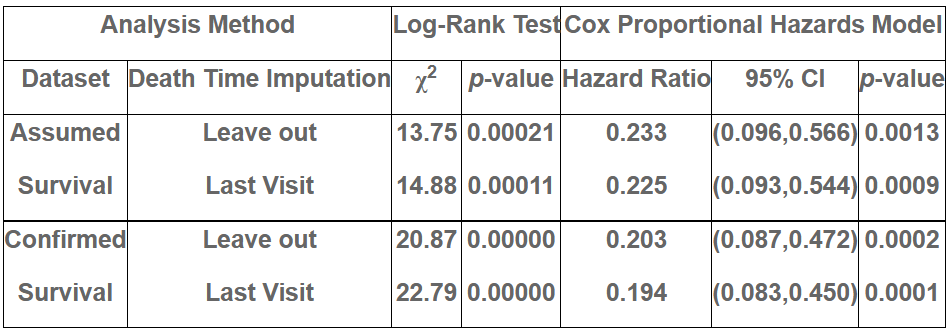
Table 1. Estimated Survival Probability by Analysis Dataset. The left column details the 4 analysis datasets considered. The middle column reports the χ2 statistic (with df = 1) and corresponding p-value from the log-rank test. The right column provides the estimated hazard ratio with 95% confidence interval and two-sided p-value.
Associate Professor Susan Mathers, Principal Investigator at Calvary Health Care Bethlehem, commented: “It has been a pleasure to be part of the development of NUZ-001 as a potential drug treatment for people living with ALS, and to work with Prof Rowe’s team at Macquarie University, Neurizon Therapeutics and especially our participants, their families and the research team at Calvary Health Care Bethlehem. NUZ-001 has been a well-tolerated therapy and we hope that the future phase 2/3 study will confirm its potential to benefit the wider ALS community.”
A detailed study presentation of the top-line results and a recorded results presentation can be accessed in the Announcements section of Neurizon’s Investor Hub at https://investorhub.neurizon.com, featuring John Clark, Chief Operating Officer, Neurizon Therapeutics, and Melanie Quintana, Ph.D., Director & Senior Statistical Scientist, Berry Consultants.
Encouraged by these results, Neurizon will advance NUZ-001 to the pivotal Phase 2/3 HEALEY ALS Platform Trial in Q4 CY2025, pending FDA clinical hold clearance, to confirm the observed benefits in a larger, placebo-controlled setting. The OLE findings reinforce the Company’s confidence in its clinical development strategy for NUZ-001 and validate the selected dosing regimen for the HEALEY ALS Platform Trial. Neurizon remains dedicated to developing NUZ-001 as a potential treatment capable of delivering meaningful clinical impact for people living with ALS.
The Company will continue to provide updates to the market as key milestones are achieved in the lead-up to anticipated trial participation in Q4 CY2025.
This announcement has been authorized for release by the Board of Neurizon Therapeutics Limited.
Neurizon Therapeutics Limited (ASX: NUZ) is a clinical-stage biotechnology company dedicated to advancing treatments for neurodegenerative diseases. Neurizon is developing its lead drug candidate, NUZ-001, for the treatment of ALS, which is the most common form of motor neurone disease. Neurizon’s strategy is to accelerate access to effective ALS treatments for patients while exploring the potential of NUZ-001 for broader neurodegenerative applications. Through international collaborations and rigorous clinical programs, Neurizon is dedicated to creating new horizons for patients and families impacted by complex neural disorders.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094


